
| Introduction | The Model (VSEPR) | Examples |

| Introduction | The Model (VSEPR) | Examples |
The electrons around the atoms in a molecule repel each other. They move to be as far apart as possible while still maintaining the bonding within the molecule. The procedure for using the model is as follows:
1) Determine the correct Lewis
structure for the molecule.
If it is a diatomic (has only two atoms) it is linear and falls into
the AX category. If it has 3 or
more atoms continue with step 2.
2) Count the number of electron groups around the central atom. A group of electrons is a bond, a nonbonding electron pair, or occasionally an unpaired nonbonding electron. Each triple or double bond counts as only one group for the purposes of this model.
3) Based on this number of groups around the central atom the
molecule falls into one of six basic
categories. Within each category there are a number of different
names for the shapes depending upon the number of atoms and nonbonding
groups around the central atom.
| Number of
Groups |
Basic Shape (click
links below to see examples)
|
Sub-Shapes A = central atom X = atom attached to central atom E = nonbonding electron group on central atom |
|
| Diatomic
(any number) |
Linear Diatomic |
Generic Formula AX |
Shape Name (this refers to the atom arrangement, ignoring the lone pairs) Linear |
| 2 |
Linear
Triatomic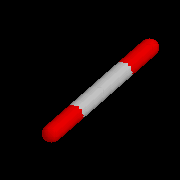 |
AX2 |
Linear |
| 3 |
Trigonal
Planar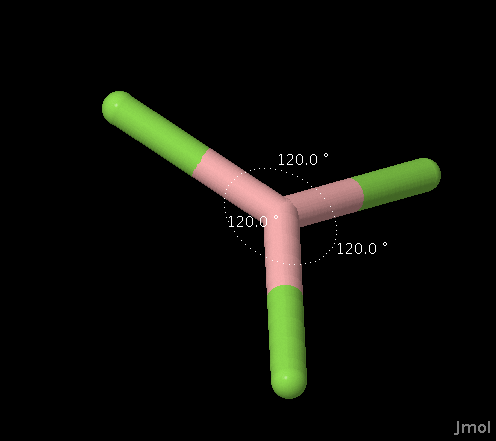 |
AX3 AX2E |
Trigonal
Planar Bent |
| 4 |
Tetrahedral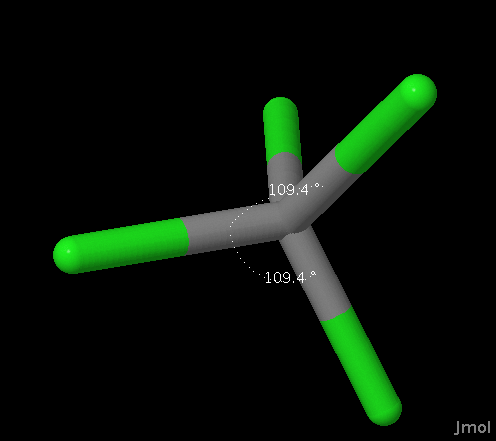 |
AX4 AX3E AX2E2 |
Tetrahedral Trigonal Pyramidal Bent |
| 5 |
Trigonal
Bipyramidal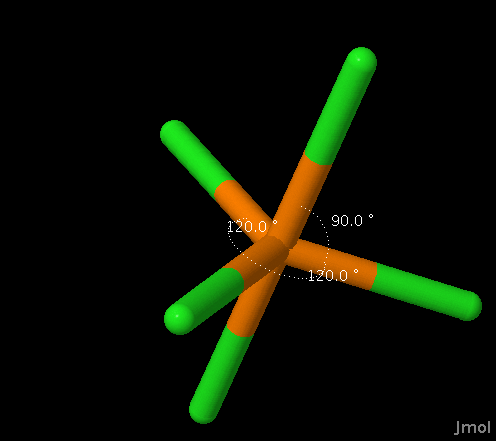 |
AX5 AX4E AX3E2 AX2E3 |
Trigonal
Bipyramidal Seesaw T-shaped Linear |
| 6 Last Table Update:
November 18, 2016
Expires: --- J. Gutow |
Octahedral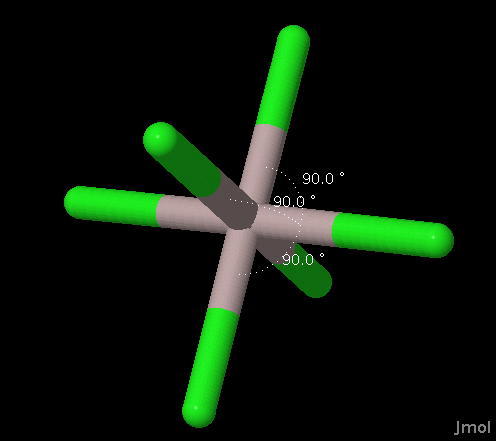 |
AX6 AX5E AX4E2 |
Octahedral Square Pyramidal Square Planar |