Once the molecule file is fully loaded, the image at right will become live. At that time the "activate 3-D" icon
![]()
will disappear.
The Bond lengths and angles are show for the highest level of
theory below due to the values for bond lengths being spot on to the
literature values obtained (Aromatic C=C at 1.39 angstroms, C-H at 1.07
angstroms, and C-Cl 1.8 angstroms. The other two levels of theory are
not shown due to the results being almost identical.
The highest occupied molecular orbital was determined to be orbital
number 37 found by adding the number of electrons and dividing by two.
Orbital 38 was the lowest unoccupied molecular orbital. This would be
occupied if the molecule received enough energy to become excited.
This is the electrostatic potential of the molecule. The red area
represents the lowest electrostatic potential and blue represents the
highest electrostatic potential.
The partial atomic charge on each atom is shown in
this diagram.
Figure 1: IR spectra of P-Dichlorobenzene
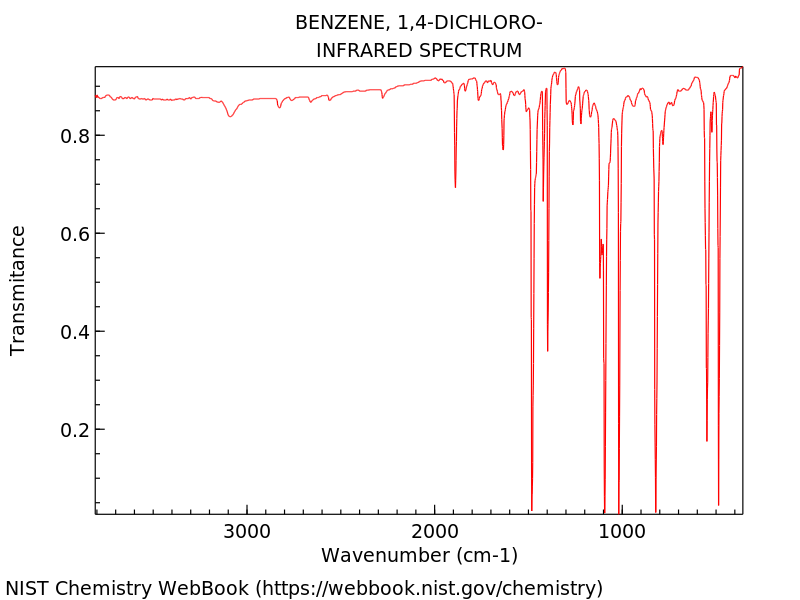
Vibrational Frequencies were calculated from the DZV level of theory.
Animations of each of the main observable vibrations are shown and
labeled with what part of the spectra they represent.
Figure 2: Experimental UV-Vis Graph
 Table 1:
Table 1: Experimental UV-Vis Peaks
The table below shows the calculated values in the DZV level of theory
for the peaks that should be observed in a UV-Vis Spectra.
Table 2: Calculated Values of UV-Vis Spectra
Oscillator Strength
(unitless)
|
Wavelength (nm)
|
0.003348
|
197.5
|
1.051193
|
147.8
|
1.617298
|
150
|
No dipole effects were observed in this molecule because of its symmetry.
Based on template by A. Herráez as modified by J. Gutow
Using directory /Users/student/Desktop/AN_Website/Pdi
adding JmolPopIn.js
...jmolApplet0
...adding Bond_Angles_and_Lengths_PDicholorobenzene_.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding Bond_Angles_and_Lengths_PDicholorobenzene_.spt
...jmolApplet1
...adding HOMO_PDicholorobenzene.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding HOMO_PDicholorobenzene.spt
...jmolApplet2
...adding LUMO_PDicholorobenzene.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding LUMO_PDicholorobenzene.spt
...jmolApplet3
...adding Electrostatic_Potential_PDicholorobenzene.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding Electrostatic_Potential_PDicholorobenzene.spt
...jmolApplet4
...adding Partial_Atomic_Charges_PDicholorobenzene.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding Partial_Atomic_Charges_PDicholorobenzene.spt
...jmolApplet5
...adding Aromatic_Bend_574_74_cm_-1_.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding Aromatic_Bend_574_74_cm_-1_.spt
...jmolApplet6
...adding Chlorine_Stretch_625_83_cm_-1_.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding Chlorine_Stretch_625_83_cm_-1_.spt
...jmolApplet7
...adding C-H_Wag_1018_96_cm_-1_.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding C-H_Wag_1018_96_cm_-1_.spt
...jmolApplet8
...adding Asymetric_C-H_Wag_1174_22_cm_-1_.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding Asymetric_C-H_Wag_1174_22_cm_-1_.spt
...jmolApplet9
...adding C-H_Oscillations_1272_02_cm_-1_.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding C-H_Oscillations_1272_02_cm_-1_.spt
...jmolApplet10
...adding C-C_Ring_Stretch_1737_42_cm_-1_.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding C-C_Ring_Stretch_1737_42_cm_-1_.spt
...jmolApplet11
...adding C-H_Ring_Stretching_3281_69_cm_-1_.png
copying and unzipping jsmol.zip directory into /Users/student/Desktop/AN_Website/Pdi
...adding C-H_Ring_Stretching_3281_69_cm_-1_.spt
![]() will disappear.
will disappear.
