Elemental chlorine is composed of two chlorine atoms. This diatomic molecule has the highest
electron affinity and is considered one of the most electronegative
elements. Given its linear structure,
chlorine is a non-polar molecule as shown below by the following Lewis
structure:

Although the above structure
depicts the correct bonding
structure of the molecule, it is not an accurate depiction according to
the
molecular orbital (MO) theory. To better understand the nature of
the
bonding and anti-bonding molecular orbitals calculations at three levels
of theory were used to determine the molecular structure These three levels were Molecular Mechanics,
MOPAC3, and Ab initio.
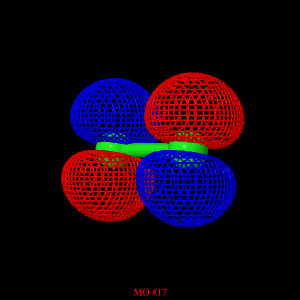
The optimized geometry and double zeta valence (DZV) basis
set were used to obtain the highest occupied molecular orbital (HOMO) for the
molecule. The blue and red surfaces above and below each sulfur atom
represent electron occupied pi-orbitals. These differences in color
represent the difference in phase for each orbital.
The figure represents the stage when the valence electrons
are in the highest energy state. Similar to the figure of the HOMO, the
lowest unoccupied molecular orbital (LUMO) is shown using the optimized
geometry and DZV basis set. Molecular orbitals are shown surrounding all
atoms in the molecule, which is consistent with the molecular orbital (MO)
theory, where the LUMO is the lowest energy state of unoccupied orbitals.
According to the MO theory, delocalized electrons become excited and transition
from the HOMO to the LUMO. This transition energy can be approximated
using the ab initio level of theory.
By clicking on the button below, a figure of the molecule can be viewed that shows a visual representation of the lowest unoccupied molecular orbital (LUMO).
The geometry of the structure shows linearity among the
atoms. This property diminishes any molecular dipole moment. The electrostatic potential represents the
changes in the potential energy in relation to the charge distribution.
By clicking on the button below, a visual representation
of the electrostatic potential field surrounding the molecule can be viewed.
As shown in the figure, the shape of the electrostatic
potential field is more elevated surround the chlorine atoms and more depressed
around the single bond. However, the
color of the field is uniform throughout, which indicates a constant potential
energy to charge distribution pattern.
The optimized geometry of the structure is in agreement with
the experimental value of bond length, 1.988 Å. Additionally, the structure is linear as
previously demonstrated by its Lewis structure.
You may look at any of these intermediate views again by clicking on the appropriate button.
References:
1.
Mihalick, J. Gutow, J. Quantum Calculations 2016, p 1-12.
2.
Chlorine. Chlorine,
http://webbook.nist.gov/cgi/cbook.cgi?id=7782-50-5 (accessed Mar 3, 2016).
3.
Carbon disulfide. Carbon disulfide,
http://webbook.nist.gov/cgi/cbook.cgi?id=75-15-0 (accessed Mar 2, 2016).
4.
Benzene, nitro-. Benzene, nitro-,
http://webbook.nist.gov/cgi/cbook.cgi?id=c98953 (accessed Mar 3, 2016).